
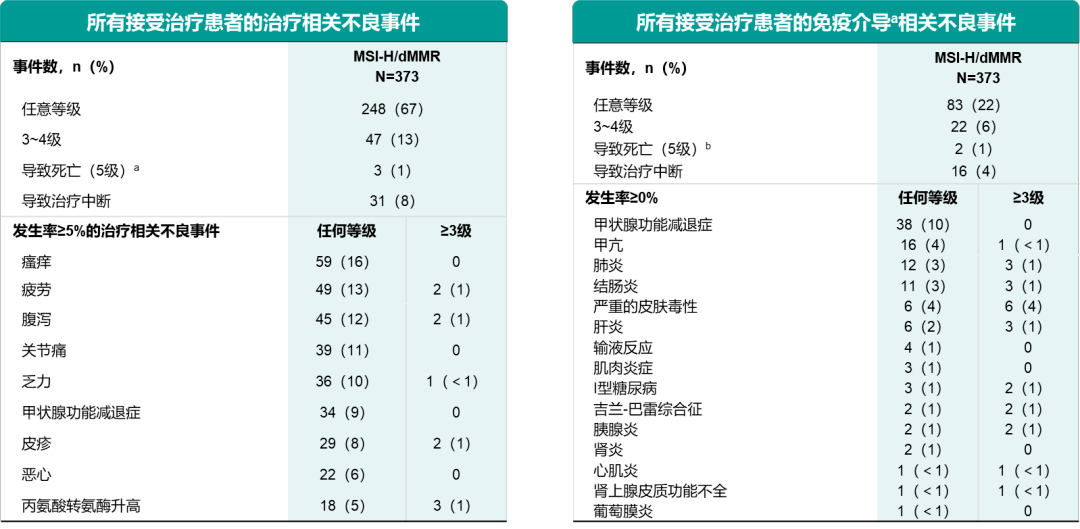
T2 - Results from the phase II KEYNOTE-158 studyĬopyright © 2019 American Society of Clinical Oncology. T1 - Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer On the basis of these results, the US Food and Drug Administration granted accelerated approval of pembrolizumab for patients with advanced PD-L1–positive cervical cancer who experienced progression during or after chemotherapy.", CONCLUSION Pembrolizumab monotherapy demonstrated durable antitumor activity and manageable safety in patients with advanced cervical cancer. Treatment-related grade 3 to 4 adverse events occurred in 12.2% of patients. Treatment-related adverse events occurred in 65.3% of patients, and the most common were hypothyroidism (10.2%), decreased appetite (9.2%), and fatigue (9.2%). Median duration of response was not reached (range, $ 3.7 to $ 18.6 months). All 12 responses were in patients with PD-L1–positive tumors, for an ORR of 14.6% (95% CI, 7.8% to 24.2%) 14.3% (95% CI, 7.4% to 24.1%) of these responses were in those who had received one or more lines of chemotherapy for recurrent or metastatic disease. ORR was 12.2% (95% CI, 6.5% to 20.4%), with three complete and nine partial responses.

Median follow-up was 10.2 months (range, 0.6 to 22.7 months). Eighty-two patients (83.7%) had programmed death-ligand 1 (PD-L1)–positive tumors (combined positive score $ 1), 77 having previously received one or more lines of chemotherapy for recurrent or metastatic disease. Median age was 46.0 years (range, 24 to 75 years), and 65.3% of patients had Eastern Cooperative Oncology Group performance status of 1. RESULTS Ninety-eight patients were treated. The primary end point was objective response rate (ORR), assessed per Response Evaluation Criteria in Solid Tumors (version 1.1) by independent central radiologic review. Tumor imaging was performed every 9 weeks for the first 12 months and every 12 weeks thereafter. PATIENTS AND METHODS Patients received pembrolizumab 200 mg every 3 weeks for 2 years or until progression, intolerable toxicity, or physician or patient decision. We present interim results from patients with previously treated advanced cervical cancer. On the basis of these results, the US Food and Drug Administration granted accelerated approval of pembrolizumab for patients with advanced PD-L1–positive cervical cancer who experienced progression during or after chemotherapy.Ībstract = "PURPOSE KEYNOTE-158 ( identifier: NCT02628067) is a phase II basket study investigating the antitumor activity and safety of pembrolizumab in multiple cancer types.


PURPOSE KEYNOTE-158 ( identifier: NCT02628067) is a phase II basket study investigating the antitumor activity and safety of pembrolizumab in multiple cancer types.


 0 kommentar(er)
0 kommentar(er)
